Expertise: Portfolio and Performance Management
Portfolio and performance management tools are some of the most critical factors of organizational success. We assist clients in creating and populating portfolio dashboards, in using such dashboards to identify areas for additional investigation and in creating corrective plans for any area of concern.
Kitterman, D, Cheng, S, Dilts DM, and Orwoll, ES, (2011) “The Prevalence and Economic Impact of Low Enrolling Clinical Studies at an Academic Medical Center,” Academic Medicine 86(11): 1360-66. PMID:21952064
Dilts DM, (2013) “A 3+1 Level Evaluation Model for CRM”, Evaluation & the Health Professions 36(4):464-477 PMID: 23908383.
Other Work Related to Portfolio and Performance Management
1. Dilts DM, Zell A, Orwoll E (2015) “A Novel Approach to Measuring Efficiency of Scientific Research Projects: Data Envelopment Analysis.” Clinical and Translational Science. 8(5):495-501. PMID:26243147
2. Perry D, Sperling R, Katz R, Berry, Dilts DM, Hanna D., … & Bens C. (2015). Building a roadmap for developing combination therapies for Alzheimer’s disease. Expert Review of Neurotherapeutics, 15(3), 327-333. PMID: 25708309
3. Stephenson D, (plus 14 others) (2015) Charting a path toward combination therapy for Alzheimer’s disease,” Expert Review of Neurotherapeutics, 15(1):107-13. PMID: 25540951
4. Dilts DM, (2013) “A 3+1 Level Evaluation Model for CRM”, Evaluation & the Health Professions 36(4):464-477 PMID: 23908383.
5. Grazier KL, Trochim W, Dilts DM, Kirk R (2013) “Estimating Return on Investment in Translational Research: Methods and Protocols”, Evaluation & the Health Professions, 36(4):478-491. PMID: 23925706
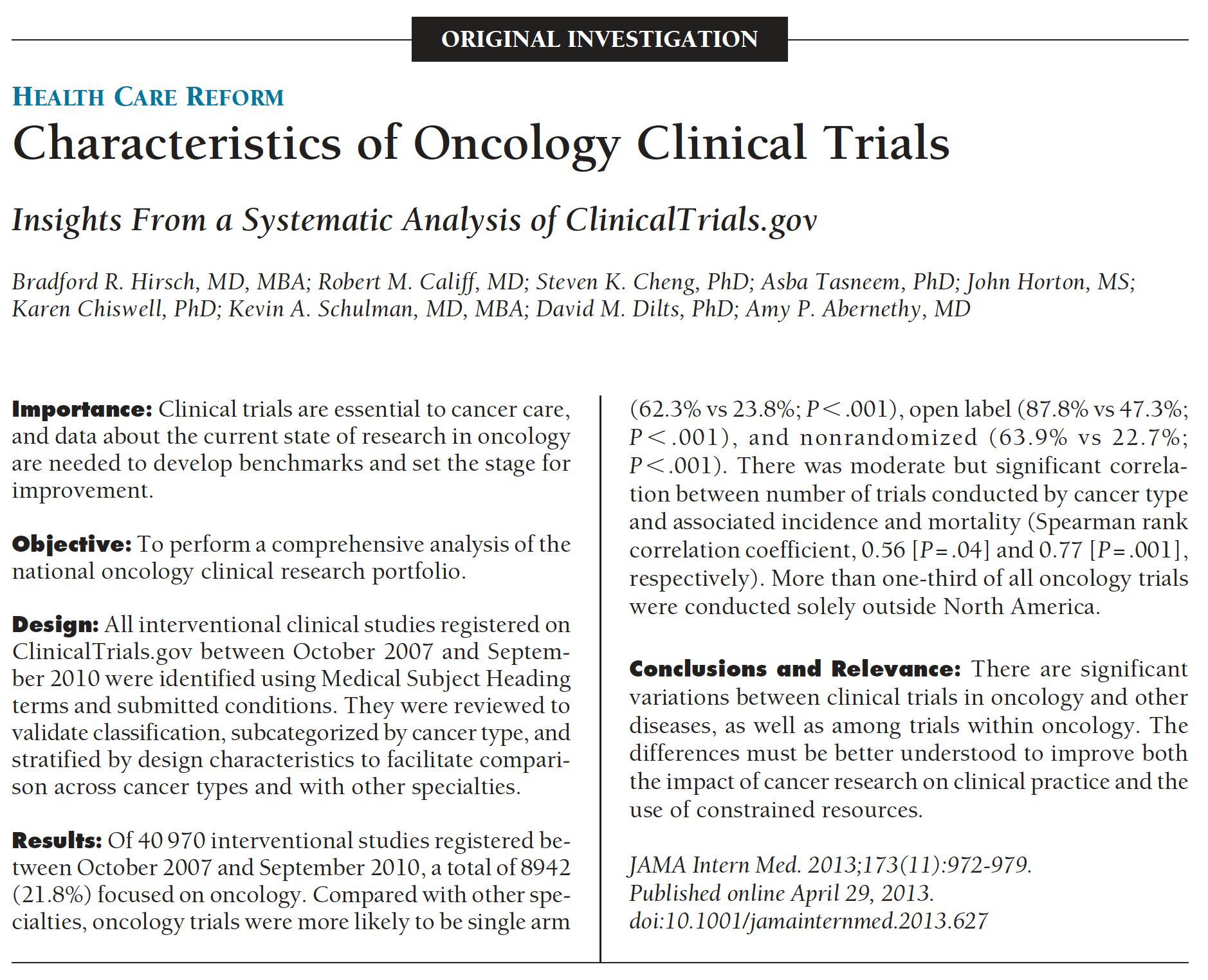
6. Hirsh BR, Califf RM, Cheng SK, Tasneem A, Horton J, Chriswell K, Schulman KA, Dilts DM, and Abernethy AP (2013) “Design of Oncology Clinical Trials: Insights from a Systematic Analysis of ClinicalTrials.gov,” JAMA Internal Medicine, 173(11):972-979. PMID:23699837.
7. Kitterman, D, Cheng, S, Dilts DM, and Orwoll, ES, (2011) “The Prevalence and Economic Impact of Low Enrolling Clinical Studies at an Academic Medical Center,” Academic Medicine 86(11): 1360-66. PMID:21952064
8. Dilts DM and Pence Ken (2006) “Impact of Role in The Decision to Fail: An Exploratory Study of Terminated Technical Projects,” J of Operations Management, 24(4): 378-396.
9. Xie Bin, Dilts DM and Shor, M (2006) “The Physician-Patient Relationship: The Impact of Patient-Obtained Medical Information,” Health Econ, 15(8): 813-833. PMID: 16550612
10. Hackett SM, and Dilts DM (2004) “A Real Options-Driven Theory of Business Incubation,” J of Technology Transfer, 29(1): 41-54. [Reprinted in “Recent Development in the Economics of Science and Innovation”, editor: A. N Link and C. Antonelli, 2013]
11. Zhang Yong and Dilts DM, (2004) “System Dynamics of Supply Chain Network Organization Structure,” Information Systems and e-Business Management: Special Issue on Supply Chain Management, 2:187-206.
12. Dilts DM (2001) “Functional Manager’s Perceptions of Performance Measurements: The Impact of Industry and Firm-Level Characteristics,” Int J of Agile Manufacturing, 4(1): 17-28.
13. Dilts DM and Khamalah J (1999) “A comparison of ordinal analysis techniques in medical resource usage research,” Electronic Notes in Discrete Mathematics. (http://www.elsevier.com/cas/tree/store/ disc/sub/endm/store/disc2/disc2005.ps) (invited), 2: 51-68
14. Doughty MJ, Dilts DM, and Lyle, WM, (1996) “Impact of Using Weighted Versus Unweighted Measures for Assessments of Optometry Faculty Productivity in Research,” J of the Am Optometric Association, 67(7): 410-420. PMID: 8888867
15. Cheney A, Pence K, and Dilts DM, (2014) “Organizational Impacts of Participation in Development of Industry-Level Technology Roadmaps,” 4th IAJC/ISAM Conference Proceedings, Sept, Orlando, FL. http://cd14.ijme.us/itc/
16. Dilts DM (2012) “Robbing Peter to Pay Paul: Financing Clinical Trial Follow-Up,” J Clin Onc, 30, PMID:22454422
17. Pathak, Surya, Dilts DM, and Biswas, Gautam, (2004) “Simulating Growth Dynamics in Complex Adaptive Supply Networks,” Winter Simulation Conference (December), pp: 774-782.
18. Dilts DM and Baik, Y (2003) “Impact of Decision Speed in the Control of an Extended Enterprise,” DSI National Meeting, (November).
19. Adamson, I. and Dilts DM, (2001) “Measurement of Accounting System Complexity at the Design Level”, Proceedings of the DSI National Meeting,.
20. Pidduck, Anne B. and Dilts DM. (1999) “Inter-Organizational Structures for Strategic Advantage,” Information Resources Management Association.
21. Dilts DM (1999) “Evaluation of Tools for Use In Determining SME Readiness for Next Generation Manufacturing, Alliance of Manufacturers and Exporters of Canada, 35 pages. (Technical Report)
22. Dilts DM (1998) “Survey of Executive Perspectives on Extended, Integrated Enterprises,” Consortium for Advanced Manufacturing – International Integrated Enterprise Technical Report, 13 pages.
23. Adamson, I. and Dilts DM, (1998) “A Hierarchy of Accounting System Complexity,” Proceedings of the DSI National Meeting,.
24. Khamalah, J. and Dilts DM (1997) “Predicting Patient Resource Needs: A Methodology for Ambulatory Care,” Proceedings of DSI National Meeting,, pp. 1464-1466.
25. Dilts DM and Wu, W, (1990) “An Intelligent Framework for the Integration of Computer Integrated Manufacturing Data Bases,” Proceedings of the DSI National Meeting, pp. 1702-1705.
26. Dilts DM and Russell, GW (1989) “Measuring External Failure: Accounting for the Four Costs of External Failure,” Proceedings of the 43rd Annual Quality Congress, American Society for Quality Control, pp. 317-322.
27. Dilts DM (1988) “Integration of Computer Integrated Manufacturing Data Bases Using Artificial Intelligence,” Second International Conference on Expert Systems and the Leading Edge in Production & Operations Management, pp. 277-284.
28. Dilts D, Cheng S, Cronin K, Fearn P, Feuer E, Friedman S, Kosary C, Lam C, Mariotto A, Negoita S, Petkov V, Penberthy L (2017) “An Innovative Approach to Developing the SEER-Wide Quality Audit Plan,” North American Association of Central Cancer Registries (NAACCR), June.
29. Massett, HA, Dilts, DM, +11 others (2016), “Will a national communication campaign to increase awareness of clinical trials work?” J Clin Oncol 34:15s (suppl; abstr: 6537)
30. Cheng SK, Hirsch BR, Califf RM, Tasneem A, Schulman KA, Abernethy AP, Dilts DM (2012) “Geographic and network analysis of oncology trials: Portfolio assessment of ClinicalTrial.gov” J Clin Oncol 30:15s (suppl; abstr 6047).
31. Hirsch BR, Califf RM, Cheng SK, Tasneem A, Chiswell K, Horton JR, Schulman KA, Dilts DM, Abernethy AP (2012) “The State of the Oncology Clinical Trials Portfolio: Insights from ClinicalTrial.gov”, Society of Clinical Trials Meeting (May).
32. Dilts DM, Peters, C, Stadum, S, Cheng, SK, “Where you stand depends upon where you sit: Alignment of quality measures to strategic intentions,” ASCO’s Quality Care Symposium, 2012.
33. Kitterman D, Cheng SK, Dilts DM, Orwoll ES (2011) “An etiologic survey of factors that contribute to non-enrolling clinical studies,” NCRR Clinical Research Management Workshop, Bethesda MD (Jun)
34. Kethert KH, Cheng SK, Nauman DJ, Dilts DM, Sandler AB, Chui SY (2011) “Implementation of a prospective screening tool for breast cancer clinical trial eligibility at an NCI designated cancer center,” J Clin Oncol 29:15s (suppl; abstr 6052)
35. Massett H, Parreco LK, Padberg RM, Richmond E, Dilts DM (2011) “AccrualNet: a novel approach to addressing clinical trial accruals through a knowledge-based, community of practice platform,” J Clin Oncol 29 (suppl; abstr e16621)
36. Dilts DM, Cheng SK, Wiltrout RH, Dahut WL, Bronez M, Helman LJ (2011) “Strategic alignment of clinical trials at the National Cancer Institute Center for Cancer Research: Development of the clinical mission and baseline portfolio metrics,” J Clin Oncol 29 (suppl; abstr e16619)
37. King A, Earnest J, Lembach J, Lycette J, Kelleher C, Dilts DM (2010) “Axis Definitions for CTSA Portfolio Analysis ,” National Center for Research Resources (NCRR), Evaluation Key Function Meeting, Bethesda MD (Dec)
38. King A, Earnest J, Lembach J, Lycette J, Kelleher C, Dilts DM (2010) “Evaluation Key Function Committee December 2010 Meeting,” National Center for Research Resources (NCRR), Evaluation Key Function Meeting, Bethesda MD (Dec)
39. Cheng S, Dietrich, M, Finnigan S, Dilts DM (2010) “Early indicators of accrual success: Time-to-first-patient and accrual performance at an anticipated enrollment milestone-A study of NCI-CTEP-sponsored clinical trials” J Clin Oncol 28:15s (suppl; abstr 6001)
40. Cheng S, Dietrich, M, Finnigan S, Sandler A, Crites J, Ferranti L, Wu A, Dilts DM (2009) “A Sense of Urgency: evaluating the link between clinical trial development time and the accrual performance of CTEP-sponsored studies,” J Clin Oncol 27:15s (suppl; abstr 6509)
41. Dilts DM, Sandler AB, Cheng S, Crites J, Ferranti L, Wu A, Bookman M, Thomas J, and Ostroff J, (2008) “Accrual to Clinical Trials at Selected Comprehensive Cancer Centers,” 26(15S): 6543
42. Dilts DM and Pence, Ken (2007) “The Decision to Fail: An Exploratory Study of Terminated Technical Projects,” Academy of Management Meeting, Philadelphia PA, best paper competition presentation for the Journal of Operations Management
43. Hackett, Sean M and Dilts DM (2006) “Real options and the option to incubate: an exploratory study of the process of business incubation,” Academy of Management Annual Meeting, Atlanta, GA.
44. Johnson, Joshua H and Dilts DM (2006) “Acquire and Forget: The conflict of information acquisition and organizational memory in the development of radical innovations,” AMA Winter Marketing Educators’ Conference.
45. Dilts DM (2006) “Applications of OM Techniques in Improving Drug Clinical Trials,” Production and Operations Management Society International Conference, Shanghai, China, June.
46. Dilts DM (2006) “Applications of OM techniques in Improving the Delivery of Healthcare” (Panel presentation) Production and Operations Management Society, Boston, April.
47. Ferranti, L and Dilts DM (2005) “A Review of Medical Registries and Outcomes: Potential Use for Real-Time Improvement in Patient Safety: The Dearth of Real-Time Science-Based Medicine,” Academy Health Annual Research and Health Policy Conference, Boston (Poster Presentation).
48. Piasecki JK, Calhoun E, Engelberg J, Rice W, Dilts DM, Belser D, Aronsky D, Jones I, Mason D, Stead B, (2005) “Computerized provider order entry in the emergency department: pilot evaluation of a return on investment analysis instrument,” AMIA Symposium Proceedings, p. 1081. PMID: 16779368
49. Inukai, Tsuyshi and Dilts DM (2004) “The Impact of Technological Innovation Type on Strategic Alliances for New Product Development,” Academy of Management National Conference.
50. Ferranti, Lori and Dilts DM (2003) “A Systematic Review of Types and Uses of Medical Registries,” 20th Annual Research Meeting of the Academy of Health. (poster) (June)
51. Dilts DM and Chuang, R. JW. (1997) “Perceptions of Service Quality in Telemedicine” Academy of Management Annual Meeting.
52. Khamalah, J, and Dilts DM, (1997) “The Application of ‘Traditional’ Manufacturing Management Techniques in Health Care Resource Usage Prediction,” Operation Management Association Forum, April.
53. (2015) “Observations on Clinical Research: Some Ideas for NHLBAC”, National Heart, Lung Blood Advisory Council, NIH,(June).
54. (2015) “Going Beyond the Norm: Some Ideas for the British Columbia Cancer Agency” British Columbia Cancer Agency Retreat, Vancouver, BC(May)
55. (2015) “Value-based Structures: Centralization and Decentralization”, NIH-Association of Biomolecular Resource Facilities Workshop on Enhancing Efficiency of Research Core Facilities, St Louis, MO(Mar)
56. (2014) “The Future of Clinical Research”, Clinical Trials Advisory Committee, Commonwealth of Australia, Sydney, AUS(Nov)
57. (2014) “Understanding the linkages among mission, processes and metrics in running a core”, Keynote address, Midwest & Southeast Association of Biomolecular Resource Facilities 2014 Annual Meeting, Nashville TN(Oct).
58. (2014) “The Promise and Perils in Clinical Trials,” Monash University, Melbourne, Australia(August, presented twice)
59. (2014) “Observations on Cancer Research from a Non-Medical Researcher,” keynote speech, Cancer Institute of New South Wales Annual Awards Banquet(August)
60. (2014) “The Importance of Doing Trials Right While doing the Right Trials,” Ministry of Health, NSW, Australia(August)
61. (2014) “Integration Metrics: Linking Mission, Science, Resources and Education,” NICHD Division of Intramural Research, Scientific Retreat, Bethesda, MD(Feb).
62. (2013) “Prioritizing and Measuring Clinical Research,” NIH Clinical Research Subcommittee, Bethesda, MD(Sept).
63. (2013) “Consortium or not to Consortium”, ACT-AD National Meeting on Alzheimer’s Research, Washington, DC(May)
64. (2013) “A Model for Evaluating the Efficiency and Effectiveness of Clinical Research,” Medical Executive Committee, NIH, Bethesda MD(May).
65. (2013) “Process Improvement: Linking Improvement to Strategy,” Oregon Health Financial Manager’s Association, Portland, OR(Feb)
66. (2012) “Precompetitive Collaboration & Coopetition: Lessons Learned from History and Other Industries,” ACT-AD annual meeting, Bethesda MD(Nov)
67. (2012) “Metrics for Clinical Trials: Lessons Learned”, CTSA Annual Evaluation Meeting, Bethesda MD(Oct)
68. (2011) “Management Principles in Healthcare” Southern Health, Melbourne AUS(Nov) and The Albert Hospital System, Melbourne AUS(Nov)
69. (2011) “Multi-disciplinary, Useful research” Melbourne Operations Management Society(MOMS)(Nov)
70. (2011) “Process Mapping – Purpose, Method & Expected Results,” University of Cincinnati Academic Health Center, Center for Clinical & Translational Science & Training(Sept)
71. (2011) “Precompetitive Collaboration: Lessons from History and Other Industries,” UCSF Accelerating Targeted Cancer Drug-Biomarker Development Conference, San Francisco CA(May)
72. (2011) “Mis-Measurement of Quality in IRBs: Thoughts from Other Industries,” 13th annual Subject Protection conference, Schulman Group, Cincinnati Children’s and the University of Kentucky, Cincinnati, OH(Sept).
73. (2010) “Clinical Trial and Regulatory Process Mapping, the Clinical Trials and Regulatory Pathways Working Group at the Center for Global Development,(Oct)
74. (2010) “Beyond Efficiency: A Strategic Approach to Clinical Research,” Keynote address, 10th Annual Oncore Users Group Madison, WI(Aug)
75. (2009) “The Need for Operations Management in Healthcare: The Case of Oncology Clinical Trials,” Distinguished Lecture, Supply Chain Management Area, Sir Wilfrid Laurier University(Dec)
76. (2009) “Envisioning the future: Lessons from the past(& present) in Oncology Clinical Trials”, keynote speech to Johns Hopkins Sidney Kimmel Comprehensive Cancer Center strategic retreat, Baltimore MD(Mar)
77. (2008) “Building Networks: Observations from Oncology and Non-Medical Industries”, keynote address for the NHLBI Workshop on Sickle Cell Disease. Washington, DC(Oct)
78. (2008) “Impact of Pathways in the Business and Technological World,” keynote address for the NCI Translates, NCI Translational Science Meeting, Washington, DC(Nov).
79. L Ferranti, PhD., Principal Investigator and V. Pinkney. Tennessee Disparity and Diversity in Public Health, “Using Data from the 2012-2013 Community Health”. CDC Poster presentation National Meeting -Assessment to Make a Difference. (April 2013).
80. Ferranti L. Vanderbilt University, School of Global Health, “The Use of Medical Registries?”. (2012)
81. Rosow CE, DiBiase PM, Zaslavsky A, Burch L. “Propofol vs. thiopental in in-patients: Comparison of recovery after general anesthesia” Proceedings of the Annual Meeting of the American Society of Anesthesiologists, Anesthesiology 1993; 79 (3): A-336.
82. Rosow CE, Burch L. “Bispectral Analysis for Monitoring Hypnotic Drug Effects” (submitted) 1999.
83. Ferranti, L. “Pediatric Asthma Compliance and Outcomes”. Paper presented at Vanderbilt University School of Nursing, Annual Research Day Nashville, TN, August, 2001.
84. Ferranti, L. “School Based Health Clinics: Their Advantages and the Challenges They Face.” Unpublished paper presented at Owen Graduate School of Management, Vanderbilt University, Nashville, TN, April, 2001.
2. Perry D, Sperling R, Katz R, Berry, Dilts DM, Hanna D., … & Bens C. (2015). Building a roadmap for developing combination therapies for Alzheimer’s disease. Expert Review of Neurotherapeutics, 15(3), 327-333. PMID: 25708309
3. Stephenson D, (plus 14 others) (2015) Charting a path toward combination therapy for Alzheimer’s disease,” Expert Review of Neurotherapeutics, 15(1):107-13. PMID: 25540951
4. Dilts DM, (2013) “A 3+1 Level Evaluation Model for CRM”, Evaluation & the Health Professions 36(4):464-477 PMID: 23908383.
5. Grazier KL, Trochim W, Dilts DM, Kirk R (2013) “Estimating Return on Investment in Translational Research: Methods and Protocols”, Evaluation & the Health Professions, 36(4):478-491. PMID: 23925706
6. Hirsh BR, Califf RM, Cheng SK, Tasneem A, Horton J, Chriswell K, Schulman KA, Dilts DM, and Abernethy AP (2013) “Design of Oncology Clinical Trials: Insights from a Systematic Analysis of ClinicalTrials.gov,” JAMA Internal Medicine, 173(11):972-979. PMID:23699837.
7. Kitterman, D, Cheng, S, Dilts DM, and Orwoll, ES, (2011) “The Prevalence and Economic Impact of Low Enrolling Clinical Studies at an Academic Medical Center,” Academic Medicine 86(11): 1360-66. PMID:21952064
8. Dilts DM and Pence Ken (2006) “Impact of Role in The Decision to Fail: An Exploratory Study of Terminated Technical Projects,” J of Operations Management, 24(4): 378-396.
9. Xie Bin, Dilts DM and Shor, M (2006) “The Physician-Patient Relationship: The Impact of Patient-Obtained Medical Information,” Health Econ, 15(8): 813-833. PMID: 16550612
10. Hackett SM, and Dilts DM (2004) “A Real Options-Driven Theory of Business Incubation,” J of Technology Transfer, 29(1): 41-54. [Reprinted in “Recent Development in the Economics of Science and Innovation”, editor: A. N Link and C. Antonelli, 2013]
11. Zhang Yong and Dilts DM, (2004) “System Dynamics of Supply Chain Network Organization Structure,” Information Systems and e-Business Management: Special Issue on Supply Chain Management, 2:187-206.
12. Dilts DM (2001) “Functional Manager’s Perceptions of Performance Measurements: The Impact of Industry and Firm-Level Characteristics,” Int J of Agile Manufacturing, 4(1): 17-28.
13. Dilts DM and Khamalah J (1999) “A comparison of ordinal analysis techniques in medical resource usage research,” Electronic Notes in Discrete Mathematics. (http://www.elsevier.com/cas/tree/store/ disc/sub/endm/store/disc2/disc2005.ps) (invited), 2: 51-68
14. Doughty MJ, Dilts DM, and Lyle, WM, (1996) “Impact of Using Weighted Versus Unweighted Measures for Assessments of Optometry Faculty Productivity in Research,” J of the Am Optometric Association, 67(7): 410-420. PMID: 8888867
15. Cheney A, Pence K, and Dilts DM, (2014) “Organizational Impacts of Participation in Development of Industry-Level Technology Roadmaps,” 4th IAJC/ISAM Conference Proceedings, Sept, Orlando, FL. http://cd14.ijme.us/itc/
16. Dilts DM (2012) “Robbing Peter to Pay Paul: Financing Clinical Trial Follow-Up,” J Clin Onc, 30, PMID:22454422
17. Pathak, Surya, Dilts DM, and Biswas, Gautam, (2004) “Simulating Growth Dynamics in Complex Adaptive Supply Networks,” Winter Simulation Conference (December), pp: 774-782.
18. Dilts DM and Baik, Y (2003) “Impact of Decision Speed in the Control of an Extended Enterprise,” DSI National Meeting, (November).
19. Adamson, I. and Dilts DM, (2001) “Measurement of Accounting System Complexity at the Design Level”, Proceedings of the DSI National Meeting,.
20. Pidduck, Anne B. and Dilts DM. (1999) “Inter-Organizational Structures for Strategic Advantage,” Information Resources Management Association.
21. Dilts DM (1999) “Evaluation of Tools for Use In Determining SME Readiness for Next Generation Manufacturing, Alliance of Manufacturers and Exporters of Canada, 35 pages. (Technical Report)
22. Dilts DM (1998) “Survey of Executive Perspectives on Extended, Integrated Enterprises,” Consortium for Advanced Manufacturing – International Integrated Enterprise Technical Report, 13 pages.
23. Adamson, I. and Dilts DM, (1998) “A Hierarchy of Accounting System Complexity,” Proceedings of the DSI National Meeting,.
24. Khamalah, J. and Dilts DM (1997) “Predicting Patient Resource Needs: A Methodology for Ambulatory Care,” Proceedings of DSI National Meeting,, pp. 1464-1466.
25. Dilts DM and Wu, W, (1990) “An Intelligent Framework for the Integration of Computer Integrated Manufacturing Data Bases,” Proceedings of the DSI National Meeting, pp. 1702-1705.
26. Dilts DM and Russell, GW (1989) “Measuring External Failure: Accounting for the Four Costs of External Failure,” Proceedings of the 43rd Annual Quality Congress, American Society for Quality Control, pp. 317-322.
27. Dilts DM (1988) “Integration of Computer Integrated Manufacturing Data Bases Using Artificial Intelligence,” Second International Conference on Expert Systems and the Leading Edge in Production & Operations Management, pp. 277-284.
28. Dilts D, Cheng S, Cronin K, Fearn P, Feuer E, Friedman S, Kosary C, Lam C, Mariotto A, Negoita S, Petkov V, Penberthy L (2017) “An Innovative Approach to Developing the SEER-Wide Quality Audit Plan,” North American Association of Central Cancer Registries (NAACCR), June.
29. Massett, HA, Dilts, DM, +11 others (2016), “Will a national communication campaign to increase awareness of clinical trials work?” J Clin Oncol 34:15s (suppl; abstr: 6537)
30. Cheng SK, Hirsch BR, Califf RM, Tasneem A, Schulman KA, Abernethy AP, Dilts DM (2012) “Geographic and network analysis of oncology trials: Portfolio assessment of ClinicalTrial.gov” J Clin Oncol 30:15s (suppl; abstr 6047).
31. Hirsch BR, Califf RM, Cheng SK, Tasneem A, Chiswell K, Horton JR, Schulman KA, Dilts DM, Abernethy AP (2012) “The State of the Oncology Clinical Trials Portfolio: Insights from ClinicalTrial.gov”, Society of Clinical Trials Meeting (May).
32. Dilts DM, Peters, C, Stadum, S, Cheng, SK, “Where you stand depends upon where you sit: Alignment of quality measures to strategic intentions,” ASCO’s Quality Care Symposium, 2012.
33. Kitterman D, Cheng SK, Dilts DM, Orwoll ES (2011) “An etiologic survey of factors that contribute to non-enrolling clinical studies,” NCRR Clinical Research Management Workshop, Bethesda MD (Jun)
34. Kethert KH, Cheng SK, Nauman DJ, Dilts DM, Sandler AB, Chui SY (2011) “Implementation of a prospective screening tool for breast cancer clinical trial eligibility at an NCI designated cancer center,” J Clin Oncol 29:15s (suppl; abstr 6052)
35. Massett H, Parreco LK, Padberg RM, Richmond E, Dilts DM (2011) “AccrualNet: a novel approach to addressing clinical trial accruals through a knowledge-based, community of practice platform,” J Clin Oncol 29 (suppl; abstr e16621)
36. Dilts DM, Cheng SK, Wiltrout RH, Dahut WL, Bronez M, Helman LJ (2011) “Strategic alignment of clinical trials at the National Cancer Institute Center for Cancer Research: Development of the clinical mission and baseline portfolio metrics,” J Clin Oncol 29 (suppl; abstr e16619)
37. King A, Earnest J, Lembach J, Lycette J, Kelleher C, Dilts DM (2010) “Axis Definitions for CTSA Portfolio Analysis ,” National Center for Research Resources (NCRR), Evaluation Key Function Meeting, Bethesda MD (Dec)
38. King A, Earnest J, Lembach J, Lycette J, Kelleher C, Dilts DM (2010) “Evaluation Key Function Committee December 2010 Meeting,” National Center for Research Resources (NCRR), Evaluation Key Function Meeting, Bethesda MD (Dec)
39. Cheng S, Dietrich, M, Finnigan S, Dilts DM (2010) “Early indicators of accrual success: Time-to-first-patient and accrual performance at an anticipated enrollment milestone-A study of NCI-CTEP-sponsored clinical trials” J Clin Oncol 28:15s (suppl; abstr 6001)
40. Cheng S, Dietrich, M, Finnigan S, Sandler A, Crites J, Ferranti L, Wu A, Dilts DM (2009) “A Sense of Urgency: evaluating the link between clinical trial development time and the accrual performance of CTEP-sponsored studies,” J Clin Oncol 27:15s (suppl; abstr 6509)
41. Dilts DM, Sandler AB, Cheng S, Crites J, Ferranti L, Wu A, Bookman M, Thomas J, and Ostroff J, (2008) “Accrual to Clinical Trials at Selected Comprehensive Cancer Centers,” 26(15S): 6543
42. Dilts DM and Pence, Ken (2007) “The Decision to Fail: An Exploratory Study of Terminated Technical Projects,” Academy of Management Meeting, Philadelphia PA, best paper competition presentation for the Journal of Operations Management
43. Hackett, Sean M and Dilts DM (2006) “Real options and the option to incubate: an exploratory study of the process of business incubation,” Academy of Management Annual Meeting, Atlanta, GA.
44. Johnson, Joshua H and Dilts DM (2006) “Acquire and Forget: The conflict of information acquisition and organizational memory in the development of radical innovations,” AMA Winter Marketing Educators’ Conference.
45. Dilts DM (2006) “Applications of OM Techniques in Improving Drug Clinical Trials,” Production and Operations Management Society International Conference, Shanghai, China, June.
46. Dilts DM (2006) “Applications of OM techniques in Improving the Delivery of Healthcare” (Panel presentation) Production and Operations Management Society, Boston, April.
47. Ferranti, L and Dilts DM (2005) “A Review of Medical Registries and Outcomes: Potential Use for Real-Time Improvement in Patient Safety: The Dearth of Real-Time Science-Based Medicine,” Academy Health Annual Research and Health Policy Conference, Boston (Poster Presentation).
48. Piasecki JK, Calhoun E, Engelberg J, Rice W, Dilts DM, Belser D, Aronsky D, Jones I, Mason D, Stead B, (2005) “Computerized provider order entry in the emergency department: pilot evaluation of a return on investment analysis instrument,” AMIA Symposium Proceedings, p. 1081. PMID: 16779368
49. Inukai, Tsuyshi and Dilts DM (2004) “The Impact of Technological Innovation Type on Strategic Alliances for New Product Development,” Academy of Management National Conference.
50. Ferranti, Lori and Dilts DM (2003) “A Systematic Review of Types and Uses of Medical Registries,” 20th Annual Research Meeting of the Academy of Health. (poster) (June)
51. Dilts DM and Chuang, R. JW. (1997) “Perceptions of Service Quality in Telemedicine” Academy of Management Annual Meeting.
52. Khamalah, J, and Dilts DM, (1997) “The Application of ‘Traditional’ Manufacturing Management Techniques in Health Care Resource Usage Prediction,” Operation Management Association Forum, April.
53. (2015) “Observations on Clinical Research: Some Ideas for NHLBAC”, National Heart, Lung Blood Advisory Council, NIH,(June).
54. (2015) “Going Beyond the Norm: Some Ideas for the British Columbia Cancer Agency” British Columbia Cancer Agency Retreat, Vancouver, BC(May)
55. (2015) “Value-based Structures: Centralization and Decentralization”, NIH-Association of Biomolecular Resource Facilities Workshop on Enhancing Efficiency of Research Core Facilities, St Louis, MO(Mar)
56. (2014) “The Future of Clinical Research”, Clinical Trials Advisory Committee, Commonwealth of Australia, Sydney, AUS(Nov)
57. (2014) “Understanding the linkages among mission, processes and metrics in running a core”, Keynote address, Midwest & Southeast Association of Biomolecular Resource Facilities 2014 Annual Meeting, Nashville TN(Oct).
58. (2014) “The Promise and Perils in Clinical Trials,” Monash University, Melbourne, Australia(August, presented twice)
59. (2014) “Observations on Cancer Research from a Non-Medical Researcher,” keynote speech, Cancer Institute of New South Wales Annual Awards Banquet(August)
60. (2014) “The Importance of Doing Trials Right While doing the Right Trials,” Ministry of Health, NSW, Australia(August)
61. (2014) “Integration Metrics: Linking Mission, Science, Resources and Education,” NICHD Division of Intramural Research, Scientific Retreat, Bethesda, MD(Feb).
62. (2013) “Prioritizing and Measuring Clinical Research,” NIH Clinical Research Subcommittee, Bethesda, MD(Sept).
63. (2013) “Consortium or not to Consortium”, ACT-AD National Meeting on Alzheimer’s Research, Washington, DC(May)
64. (2013) “A Model for Evaluating the Efficiency and Effectiveness of Clinical Research,” Medical Executive Committee, NIH, Bethesda MD(May).
65. (2013) “Process Improvement: Linking Improvement to Strategy,” Oregon Health Financial Manager’s Association, Portland, OR(Feb)
66. (2012) “Precompetitive Collaboration & Coopetition: Lessons Learned from History and Other Industries,” ACT-AD annual meeting, Bethesda MD(Nov)
67. (2012) “Metrics for Clinical Trials: Lessons Learned”, CTSA Annual Evaluation Meeting, Bethesda MD(Oct)
68. (2011) “Management Principles in Healthcare” Southern Health, Melbourne AUS(Nov) and The Albert Hospital System, Melbourne AUS(Nov)
69. (2011) “Multi-disciplinary, Useful research” Melbourne Operations Management Society(MOMS)(Nov)
70. (2011) “Process Mapping – Purpose, Method & Expected Results,” University of Cincinnati Academic Health Center, Center for Clinical & Translational Science & Training(Sept)
71. (2011) “Precompetitive Collaboration: Lessons from History and Other Industries,” UCSF Accelerating Targeted Cancer Drug-Biomarker Development Conference, San Francisco CA(May)
72. (2011) “Mis-Measurement of Quality in IRBs: Thoughts from Other Industries,” 13th annual Subject Protection conference, Schulman Group, Cincinnati Children’s and the University of Kentucky, Cincinnati, OH(Sept).
73. (2010) “Clinical Trial and Regulatory Process Mapping, the Clinical Trials and Regulatory Pathways Working Group at the Center for Global Development,(Oct)
74. (2010) “Beyond Efficiency: A Strategic Approach to Clinical Research,” Keynote address, 10th Annual Oncore Users Group Madison, WI(Aug)
75. (2009) “The Need for Operations Management in Healthcare: The Case of Oncology Clinical Trials,” Distinguished Lecture, Supply Chain Management Area, Sir Wilfrid Laurier University(Dec)
76. (2009) “Envisioning the future: Lessons from the past(& present) in Oncology Clinical Trials”, keynote speech to Johns Hopkins Sidney Kimmel Comprehensive Cancer Center strategic retreat, Baltimore MD(Mar)
77. (2008) “Building Networks: Observations from Oncology and Non-Medical Industries”, keynote address for the NHLBI Workshop on Sickle Cell Disease. Washington, DC(Oct)
78. (2008) “Impact of Pathways in the Business and Technological World,” keynote address for the NCI Translates, NCI Translational Science Meeting, Washington, DC(Nov).
79. L Ferranti, PhD., Principal Investigator and V. Pinkney. Tennessee Disparity and Diversity in Public Health, “Using Data from the 2012-2013 Community Health”. CDC Poster presentation National Meeting -Assessment to Make a Difference. (April 2013).
80. Ferranti L. Vanderbilt University, School of Global Health, “The Use of Medical Registries?”. (2012)
81. Rosow CE, DiBiase PM, Zaslavsky A, Burch L. “Propofol vs. thiopental in in-patients: Comparison of recovery after general anesthesia” Proceedings of the Annual Meeting of the American Society of Anesthesiologists, Anesthesiology 1993; 79 (3): A-336.
82. Rosow CE, Burch L. “Bispectral Analysis for Monitoring Hypnotic Drug Effects” (submitted) 1999.
83. Ferranti, L. “Pediatric Asthma Compliance and Outcomes”. Paper presented at Vanderbilt University School of Nursing, Annual Research Day Nashville, TN, August, 2001.
84. Ferranti, L. “School Based Health Clinics: Their Advantages and the Challenges They Face.” Unpublished paper presented at Owen Graduate School of Management, Vanderbilt University, Nashville, TN, April, 2001.